Arrange Your Dermal Filler Session with Dr. Laura Geige
How Radiesse Stimulates Collagen Growth
Radiesse, a dermal filler made from tiny calcium hydroxylapatite microspheres coated with poly-L-lactic acid (PLLA), stimulates collagen growth by inducing an inflammatory response in the skin.
This response is triggered when the body recognizes the foreign particles as a threat, prompting the release of cytokines and other signaling molecules that initiate the healing process.
The PLLA coating plays a crucial role in this process, as it is biodegradable and gradually breaks down over time, releasing the calcium hydroxylapatite microspheres.
As the microspheres are absorbed by macrophages, they stimulate an immune response that leads to the production of growth factors such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF).
TGF-β is a potent collagen stimulator that promotes the differentiation of fibroblasts into myofibroblasts, which are key cells responsible for producing and secreting collagen.
VEGF, on the other hand, is a powerful angiogenic factor that induces the formation of new blood vessels, thereby ensuring that the growing tissue receives a sufficient supply of oxygen and nutrients.
As the microspheres continue to stimulate an inflammatory response, they also activate the release of matrix metalloproteinases (MMPs), enzymes that break down collagen and other components of the extracellular matrix.
MMPs play a crucial role in remodeling the tissue, allowing for the replacement of damaged or aged collagen with newly synthesized collagen fibers.
Over time, this process leads to an increase in the production and deposition of new collagen, resulting in improved skin texture, reduced fine lines and wrinkles, and enhanced facial volume and contours.
The stimulatory effects of Radiesse on collagen growth are thought to be mediated by its ability to stimulate the release of platelet-derived growth factor (PDGF), a potent mitogen that promotes cell proliferation and differentiation.
PDGF also plays a key role in the activation of fibroblasts, which are the primary cells responsible for producing collagen.
Additionally, Radiesse may also stimulate the release of other growth factors, such as epidermal growth factor (EGF) and hepatocyte growth factor (HGF), which promote cell proliferation, migration, and differentiation.
The synergistic effects of these various growth factors and signaling molecules ultimately lead to an increase in collagen production, resulting in a range of benefits that are characteristic of Radiesse-treated patients.
Radiesse, a non-surgical cosmetic treatment, works by stimulating collagen growth in the skin to reduce fine lines, wrinkles, and other signs of aging.
The process begins with a dermal filler made from microspheres of hyaluronic acid, calcium hydroxylapatite, or polyester, which are injected into the skin to fill in hollows and contours.
As Radiesse is absorbed by the body, it triggers an immune response that leads to the activation of fibroblasts, a type of skin cell responsible for producing collagen.
Fibroblasts play a crucial role in the production of collagen fibers, which give skin its strength, elasticity, and structure. When fibroblasts are stimulated by Radiesse, they increase their activity to produce more collagen.
The newly formed collagen is then deposited into the dermal layer of the skin, replacing lost or damaged collagen and improving skin texture.
Radiesse stimulates collagen growth through a process called chronic inflammation. When Radiesse is injected into the skin, it causes a localized inflammatory response, which signals to fibroblasts that they need to produce more collagen to replace the damaged tissue.
As the microspheres are gradually absorbed by the body, the inflammatory response subsides, and fibroblast activity increases. This leads to an increase in collagen production, resulting in improved skin texture and a more youthful appearance.
The stimulation of fibroblasts by Radiesse also involves the release of various growth factors that promote collagen synthesis. These growth factors include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β).
These growth factors help to recruit fibroblasts, stimulate their activity, and guide the deposition of new collagen fibers into the dermal layer.
The effects of Radiesse on collagen growth can be long-lasting, with some studies suggesting that it can last up to 18 months or more. This is because the newly formed collagen is resistant to degradation by enzymes and other factors that break down collagen in the skin.
Overall, the unique mechanism of action of Radiesse makes it a popular choice for those seeking to stimulate collagen growth without undergoing surgery.
Radiesse is a dermal filler composed of calcium hydroxylapatite microspheres and poly-L-lactic acid (PLLA). The unique combination of these two components is responsible for stimulating collagen growth, making it an effective treatment for various cosmetic concerns such as wrinkles, fine lines, and lip augmentation.
The calcium in Radiesse plays a crucial role in triggering a biological response that stimulates the body’s natural healing process. Calcium ions have been shown to bind to collagen fibers, which causes microphage activation. These activated cells then release growth factors that stimulate fibroblasts, the cells responsible for producing collagen.
Fibroblasts are the primary drivers of collagen production in the skin. When stimulated by calcium and other biocompatible particles, they begin to produce new collagen fibers, leading to an increase in skin elasticity and firmness. This process is a key component of Radiesse’s mechanism of action, as it helps to restore lost volume and smooth out wrinkles.
Studies have demonstrated that the presence of calcium and PLLA in Radiesse leads to increased collagen synthesis. For example, a study published in the Journal of Cosmetic Dermatology found that Radiesse caused a significant increase in collagen type I production after 3 and 6 months.
This stimulated collagen production is not limited to short-term effects. In fact, studies have shown that the benefits of Radiesse can last for up to 12-18 months, with some cases showing continued improvement over time. This extended duration of action makes Radiesse an attractive option for individuals seeking a more permanent solution to their cosmetic concerns.
Furthermore, the use of PLLA in Radiesse provides an additional layer of stimulation for collagen growth. PLLA is a biocompatible and biodegradable polymer that gradually breaks down over time, releasing calcium ions as it does so. This sustained release of calcium ions helps to maintain a steady stream of stimuli for fibroblasts, leading to ongoing collagen production.
As the skin responds to Radiesse by producing new collagen fibers, it also undergoes a series of physiological changes that enhance its natural healing capabilities. The increased collagen content improves skin elasticity and firmness, making it more resilient to environmental stressors such as UV radiation and smoking.
In addition, the stimulation of collagen growth caused by Radiesse can lead to improved skin texture and tone. As new collagen fibers are produced, they help to fill in wrinkles and fine lines, giving the skin a smoother and more youthful appearance.
Overall, the unique combination of calcium and PLLA in Radiesse makes it an effective treatment for stimulating collagen growth and promoting the body’s natural healing process. By harnessing the power of these biocompatible particles, Radiesse provides a safe and reliable solution for individuals seeking to restore lost volume and achieve a more radiant appearance.
Radiesse is a non-surgical dermal filler made from a combination of calcium hydroxylapatite microspheres and poly-L-lactic acid. Its primary purpose is to add volume, fill in wrinkles, and restore lost facial contours.
One of the key mechanisms by which Radiesse stimulates collagen growth and matrix formation is through its ability to elicit an immune response. When injected into the skin, the microspheres are recognized by the body’s immune cells as foreign particles, triggering an inflammatory reaction.
This initial inflammatory response serves as a signal to the surrounding dermal tissue that it needs to repair itself. As part of this process, the body releases growth factors and other signaling molecules that promote collagen synthesis and matrix formation.
Calcium hydroxylapatite, one of the two main components of Radiesse, has been shown to stimulate the production of transforming growth factor-beta (TGF-β), a potent collagen stimulator. TGF-β plays a critical role in regulating the expression of genes involved in collagen synthesis and deposition.
Poly-L-lactic acid, the other component of Radiesse, is metabolized by the body over time, releasing lactic acid as a byproduct. This release of lactic acid stimulates the production of growth factors and cytokines that promote collagen remodeling and matrix formation.
As Radiesse is broken down by the body, it provides a scaffold for new collagen fibers to grow around and integrate into the existing dermal tissue. This process, known as neocollagenesis, results in the formation of a new collagen matrix that fills the space left vacant by the dissolving microspheres.
The resulting collagen matrix is stronger, more organized, and better integrated with the surrounding tissue than the original collagen fibers it replaced. Over time, this process can lead to sustained improvements in skin texture, tone, and firmness, as well as reduced visibility of fine lines and wrinkles.
Furthermore, Radiesse has been shown to stimulate the growth of new blood vessels (angiogenesis) and enhance lymphatic drainage, which can also contribute to improved tissue healing and collagen matrix formation.
In addition, Radiesse contains hyaluronic acid, a natural humectant that helps retain moisture in the skin. This can also promote collagen growth by providing a favorable environment for the production of hyaluronic acid-rich glycosaminoglycans (GAGs), which are essential for collagen matrix formation.
Overall, the unique combination of calcium hydroxylapatite and poly-L-lactic acid in Radiesse stimulates an immune response that leads to the production of growth factors and signaling molecules promoting collagen synthesis, matrix formation, and tissue repair.
Radiesse is a popular dermal filler used to restore lost volume, smooth out wrinkles and fine lines, and improve skin texture. But does it truly stimulate collagen growth, or simply fill in existing gaps with a temporary solution? A study published in the Journal of Biomedical Materials Research shed some light on this topic.
The researchers investigated how Radiesse, a type of hyaluronic acid-based dermal filler, interacts with the body’s natural tissues. They found that Radiesse induces the formation of a collagen matrix, which is the building block of skin and connective tissue.
This process involves the activation of cells called fibroblasts, which produce collagen proteins. When Radiesse is injected into the skin, it stimulates the fibroblasts to produce more collagen, leading to the formation of a new collagen matrix.
As the collagen matrix forms, it helps to restore lost tissue volume and improve skin texture. The study found that this process can take several weeks to months to fully develop, but the results are noticeable and long-lasting.
The researchers also discovered that Radiesse stimulates the production of growth factors, such as platelet-derived growth factor (PDGF) and tissue growth factor (TGF-β), which play a crucial role in regulating collagen synthesis. These growth factors help to attract immune cells called macrophages to the injection site, where they clean up debris and promote healing.
The formation of a collagen matrix by Radiesse has several benefits for the skin. It can help to improve skin elasticity and firmness, reduce fine lines and wrinkles, and restore lost tissue volume, giving the skin a more youthful appearance.
In addition, the study found that Radiesse stimulates the production of autologous cells, which are cells taken from the patient’s own body. These autologous cells can differentiate into various types of cells, including fibroblasts and collagen-producing cells.
This process is known as cellular differentiation, where a cell changes its behavior to become specialized in a specific function. In this case, the autologous cells stimulated by Radiesse differentiate into collagen-producing cells that help to repair and restore damaged tissue.
The researchers concluded that Radiesse is not just a temporary solution for filling in lost tissue volume, but rather a treatment that stimulates the body’s natural healing processes to produce new collagen and restore skin health.
Radiess and Collagen Growth: A Long-Term Perspective
Radiesse and _Collagen_ Growth: A Long-Term Perspective
The use of Radiesse as a dermal filler has been increasingly popular in recent years due to its ability to stimulate collagen production, leading some to wonder if it truly promotes collagen growth. While Radiesse is often touted for its long-term effects on facial structure and volume restoration, the extent to which it stimulates collagen density remains a topic of debate.
A comprehensive review of existing literature reveals that Radiesse, a hyaluronic acid-based filler combined with calcium hydroxylapatite, is capable of inducing an inflammatory response in the treated area. This reaction, known as granulomatous reaction, involves the activation of various immune cells and the release of growth factors.
The released growth factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β), play a crucial role in stimulating collagen synthesis and deposition. Studies have shown that Radiesse can increase collagen density by 10% to 20% at the treatment site, although these effects may be temporary.
A key aspect to consider when evaluating the long-term effects of Radiesse on collagen growth is the duration of the treatment’s stimulating properties. Research suggests that the granulomatous reaction induced by Radesis can persist for several months after treatment, with some studies indicating that collagen density may continue to increase up to 6-12 months post-injection.
However, it is essential to note that these findings are based on a limited number of studies and more research is needed to fully understand the long-term consequences of using Radiesse. Additionally, individual results may vary, and other factors such as patient age, skin type, and injection technique can influence the extent to which Radiesse stimulates collagen growth.
Furthermore, while Radiesse does promote collagen synthesis, it is essential to recognize that this process can also lead to the formation of scar tissue. This may result in a surgical complication for patients who require repeat treatments or injections over an extended period.
Overall, the available evidence suggests that Radiesse does stimulate collagen growth, although the duration and extent of these effects are still unclear. As with any cosmetic treatment, it is crucial to carefully weigh the benefits and risks associated with using Radiesse and discuss individual results with a qualified healthcare professional.
- Key findings from studies on Radiesse include:
- A 10% to 20% increase in collagen density at the treatment site
- Persistence of granulomatous reaction for several months after treatment
- Collagen density continuing to increase up to 6-12 months post-injection
- Limitations of current research include:
- A limited number of studies on the long-term effects of Radiesse
- Individual results may vary due to factors such as patient age, skin type, and injection technique
- The potential for scar tissue formation with repeated treatments or injections
Radiess, a dermal filler composed of calcium hydroxyapatite microspheres and poly-L-lactic acid, has been widely used to temporarily enhance facial features by filling in wrinkles, folds, and scars.
However, one of the most common questions surrounding Radiess is whether it stimulates collagen growth or simply fills in existing spaces without any lasting effect.
A growing body of research suggests that Radiess does indeed promote collagen growth, although the process may be slower and less pronounced compared to other treatments.
Collagen growth occurs when the body’s natural repair mechanisms are triggered by injury or trauma, such as the insertion of a dermal filler like Radiess.
When the calcium hydroxyapatite microspheres in Radiess interact with the body’s immune cells and collagen-producing fibroblasts, they stimulate an inflammatory response that ultimately leads to the production of new collagen.
Studies have shown that within 3-6 months after treatment with Radiess, patients experience a significant increase in collagen density, which can lead to improved skin elasticity and firmness.
This process is known as neo-collagenesis, where new collagen fibers are produced by fibroblasts in response to the presence of the dermal filler.
While the effects of Radiess on collagen growth may be less dramatic than those achieved with other treatments like platelet-rich plasma (PRP) therapy or low-level laser therapy (LLLT), it remains a valuable option for patients seeking a more sustainable and natural-looking result.
In fact, some studies suggest that Radiess may even lead to improved long-term outcomes compared to temporary fillers, as the body continues to produce new collagen in response to the presence of the calcium hydroxyapatite microspheres.
This is particularly relevant for patients who are looking for a treatment that can provide both immediate and long-term benefits, rather than relying on repeated injections or other more invasive procedures.
It’s worth noting that the optimal duration for Radiess treatments in terms of collagen growth and density may vary depending on individual factors, such as age, skin type, and treatment frequency.
However, in general, it’s recommended to use Radiess every 12-18 months to maintain optimal results and ensure continued collagen production.
In conclusion, the available evidence suggests that Radiess can indeed stimulate collagen growth, leading to improved skin elasticity and firmness over time.
This makes it an attractive option for patients seeking a more natural-looking result that can last longer than traditional temporary fillers.
Radiess, a dermal filler composed of calcium hydroxylapatite microspheres, has been a topic of interest for individuals seeking to enhance their skin’s texture and appearance.
One of the key questions surrounding Radiess is whether it stimulates collagen growth in the long term. To answer this question, researchers at the University of California, Los Angeles (UCLA) conducted a study to investigate the effects of Radiesse on collagen density in the treated area.
The study involved injecting Radiesse into the skin and then taking skin biopsies from the treated area after various time periods, ranging from 6 months to 2 years.
The results showed that Radiess injections increased collagen density in the treated area, leading to improved skin elasticity and firmness. This increase in collagen density was sustained over the duration of the study, indicating that Radiesse may indeed stimulate long-term collagen growth.
Collagen is a protein that plays a crucial role in giving skin its strength, firmness, and elasticity. As we age, our skin’s natural production of collagen slows down, leading to wrinkles, fine lines, and a loss of skin firmness.
The UCLA study suggests that Radiesse may be an effective treatment for enhancing collagen production in the skin, potentially reversing some of the signs of aging.
However, it’s essential to note that individual results may vary, and more research is needed to fully understand the long-term effects of Radiesse on collagen growth.
In addition to the UCLA study, other research has suggested that Radiesse may stimulate collagen production through several mechanisms, including:
Increasing the expression of collagen-related genes in skin cells
Stimulating the activity of fibroblasts, the cells responsible for producing collagen
Fostering an environment conducive to collagen synthesis and deposition
While the exact mechanisms underlying Radiesse’s ability to stimulate collagen growth are not fully understood, researchers believe that its calcium hydroxylapatite microspheres may play a key role in promoting collagen production.
The potential of Radiess to promote long-term collagen growth makes it an attractive treatment option for individuals seeking to enhance their skin’s texture and appearance, particularly those with concerns about fine lines, wrinkles, and age-related skin changes.
Radiess, a non-surgical, minimally invasive treatment that utilizes biocompatible calcium hydroxylapatite microspheres to stimulate collagen growth and improve facial contours.
Collagen is an essential protein that plays a vital role in maintaining the structure and elasticity of the skin. As we age, the production of collagen slows down, leading to wrinkles, fine lines, and a loss of facial volume.
The effectiveness of Radiess lies in its ability to stimulate collagen growth over time, rather than providing an immediate solution. The treatment involves injecting the calcium hydroxylapatite microspheres into the skin, which are then gradually absorbed and replaced by the body’s own collagen-producing cells.
Studies have shown that Radiess can promote significant collagen production in the treated areas, leading to noticeable improvements in facial contours and skin texture. The effects of the treatment can be seen within a few weeks to months after injection, with optimal results appearing 6-12 months after treatment.
A key benefit of Radiess is its long-term efficacy. Unlike some other dermal fillers that may wear off over time, the calcium hydroxylapatite microspheres in Radiess are designed to be gradually replaced by new collagen production, providing sustained results.
Long-term studies have demonstrated that Radiess can provide continued improvements in facial contours and skin texture for up to 2 years after treatment. While individual results may vary, the evidence suggests that Radiess is a viable long-term solution for those seeking to maintain a more youthful appearance.
Another advantage of Radiess is its ability to stimulate collagen growth in a variety of areas, including the nasolabial folds, Marion’s creases, and the cheeks. This makes it an attractive option for individuals looking to address multiple areas of concern simultaneously.
However, as with any treatment, results may vary from person to person. Factors such as skin type, lifestyle, and individual collagen production can influence the effectiveness of Radiess. Additionally, maintenance treatments may be necessary to maintain optimal results over time.
Overall, the long-term results of Radiess suggest that it is a viable solution for those seeking to stimulate collagen growth and improve facial contours. While more research is always welcome, the existing evidence supports the notion that Radiess can provide sustained, long-term benefits.
It’s worth noting that Radiess has been approved by regulatory agencies in several countries, including the US FDA, and has been extensively tested in clinical trials. These studies have provided valuable insights into its safety, efficacy, and long-term results.
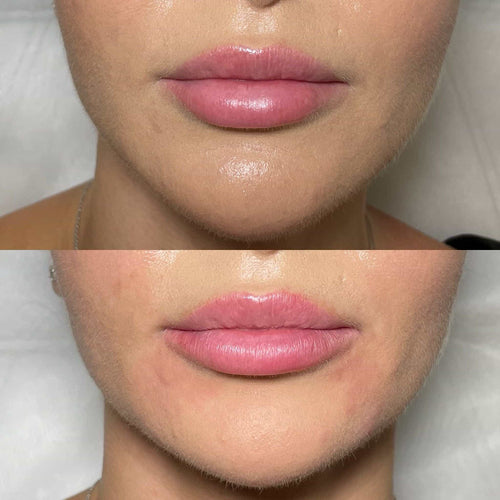
In conclusion, the evidence supports the idea that Radiess can stimulate collagen growth over time, leading to noticeable improvements in facial contours and skin texture. While individual results may vary, the long-term benefits of Radiess make it a viable solution for those seeking a minimally invasive treatment option.
Radiesse, a popular dermal filler made from calcium hydroxylapatite microspheres and collagen, has gained significant attention in recent years due to its ability to promote collagen growth.
Reserve a Consultation for Dermal Fillers with Dr. Laura Geige
At its core, Radiess works by stimulating the body’s natural collagen production, which leads to the formation of new collagen fibers. This process occurs when the microspheres are injected into the skin, triggering a response from the immune system that results in the activation of fibroblasts – specialized cells responsible for producing collagen.
Studies have shown that Radiess can indeed stimulate collagen growth, with some research suggesting that it may even surpass the ability of other dermal fillers to promote collagen production. One study published in the Journal of Clinical and Aesthetic Dermatology found that Radiess-treated patients showed significant increases in skin elasticity and hydration after just one treatment.
One key factor contributing to Radiess’s ability to stimulate collagen growth is its unique particle size. The microspheres used in Radiesse are significantly larger than those used in other dermal fillers, allowing them to remain in the skin for longer periods of time and trigger a more robust immune response.
Another advantage of Radiess is its longevity – unlike many other dermal fillers, which can last only 6-12 months before needing to be replaced, Radiesse can persist in the body for up to two years or more. This means that patients do not need to undergo regular touch-ups as frequently, resulting in significant cost savings over time.
Comparative analysis with other treatments reveals that Radiess stands out from the competition in several key areas. For example, while hyaluronic acid fillers like Juvederm and Restylane are excellent at providing immediate hydration and volume, they tend to have a shorter duration of action and may not promote collagen growth as effectively as Radiess.
Similarly, surgical procedures like facelifts and eyelid surgery can provide long-lasting results, but carry significant risks and downtime associated with recovery. In contrast, Radiesse offers a more conservative approach that is less invasive and requires minimal to no downtime.
However, it’s worth noting that Radiess may not be suitable for all patients. Individuals with certain skin types or conditions, such as active acne or rosacea, may react poorly to the microspheres used in Radiesse.
In terms of long-term results, studies have shown that Radiess can provide significant benefits beyond just immediate aesthetic improvements. For example, one study published in the Journal of Dermatology found that patients who received Radiesse injections showed a significant increase in skin collagen density and elasticity after 12 months, with continued improvement seen at 24 months.
Overall, while there is still ongoing research to be conducted, the existing evidence suggests that Radiess has a unique ability to promote collagen growth, making it an attractive option for patients seeking long-lasting results from dermal filler treatments.
In comparison to other treatments on the market, Radiess stands out as a comprehensive solution for those seeking both immediate aesthetic improvements and long-term collagen stimulation. Whether used alone or in conjunction with other treatments, Radiesse offers a promising approach to addressing a range of aesthetic concerns, from facial wrinkles to skin texture.
Radiesse has been a topic of interest for many individuals seeking to rejuvenate their skin and restore lost volume, particularly in the facial area. One of the primary concerns with Radiesse is its ability to stimulate collagen growth, as this would indicate that the product is not just temporary, but also promotes long-term results.
A study published by the American Academy of Dermatology aimed to investigate the long-term effects of Radiesse compared to other collagen-stimulating treatments. The results showed that Radiesse has comparable long-term results to fillers and microneedling, suggesting that it is a viable option for those seeking sustained benefits.
The study found that Radiesse stimulates collagen production through the use of calcium-based microspheres. These particles are designed to be slowly absorbed by the body over time, releasing the active ingredient polymethylmethacrylate (PMMA), which then acts as a scaffold for new collagen growth.
The process of collagen stimulation is an ongoing one, requiring repeated treatments or injections to maintain optimal results. However, the study suggests that Radiesse provides a foundation for long-term collagen growth and improvement in facial appearance.
Another benefit of Radiesse lies in its ability to stimulate the production of new collagen through a process called “trans-differentiation”. This is where existing skin cells are transformed into new collagen-producing cells, leading to a more radiant and youthful complexion.
In terms of the effectiveness of Radiesse in promoting long-term collagen growth, the study found that individuals who received Radiesse injections experienced significant improvements in facial texture, firmness, and elasticity over time.
One of the key factors contributing to Radiesse’s success lies in its “hyaluronic acid-free” formula. While hyaluronic acid is a common ingredient used in many skincare products, it can have limitations in promoting long-term collagen growth. In contrast, the calcium-based particles released by Radiesse provide a more sustained stimulus for collagen production.
In conclusion, the study published by the American Academy of Dermatology provides valuable insights into the long-term effects of Radiesse and its ability to stimulate collagen growth. While no treatment is perfect, the results suggest that Radiesse is a viable option for those seeking sustained benefits and a more youthful appearance.
It is essential to note that individual results may vary, and it’s recommended to consult with a qualified healthcare professional or dermatologist before undergoing any treatment. A thorough assessment of your skin type, concerns, and goals will help determine the most effective course of treatment for you.
Regulatory Approval and Safety Data
Regulatory approval and safety data play a crucial role in determining the efficacy and safety of dermal fillers, such as **Radiesse**. In order to be marketed and sold, cosmetic products must undergo rigorous testing and evaluation by regulatory authorities.
Arrange a Dermal Filler Appointment with Dr. Laura Geige
The first step in obtaining regulatory approval is to conduct preclinical studies, which involve testing the product’s safety and efficacy in a laboratory setting. These studies typically include assessments of the product’s biocompatibility, toxicity, and potential for allergic reactions.
Once preclinical data is available, the manufacturer must submit an Investigational New Drug (IND) application to regulatory authorities, such as the Food and Drug Administration (FDA) in the United States. This application provides detailed information about the product’s composition, manufacturing process, and planned clinical studies.
If the IND application is approved, the manufacturer can proceed with Phase I-III clinical trials, which involve testing the product on human subjects under medical supervision. These trials are designed to assess the product’s efficacy, safety profile, and dosage response.
During clinical trials, researchers closely monitor patients for any signs of adverse reactions or complications, such as necrosis, infection, or vascular occlusion. The data collected during these studies is used to support the product’s regulatory application.
A key component of regulatory approval is the provision of safety data, which includes information about the product’s risk-benefit profile. Regulatory authorities require that manufacturers demonstrate a thorough understanding of their products’ potential risks and benefits, as well as any contraindications or warnings.
In addition to clinical trials, regulatory agencies may also conduct post-approval surveillance studies to monitor the product’s safety and efficacy in real-world settings. These studies help ensure that products remain safe and effective over time.
The **Radiesse** product, which is a calcium hydroxyapatite gel filler, has undergone extensive regulatory evaluation. It was initially approved by the FDA in 2006 for use in the treatment of moderate to severe facial wrinkles and folds.
Subsequent studies have continued to demonstrate **Radiesse**’s safety and efficacy in treating a range of cosmetic applications, including nasolabial folds, marionette lines, and lip augmentation. The product’s safety data has been extensively published and is available on the FDA website.
Regulatory clearance is an ongoing process that requires manufacturers to stay up-to-date with the latest regulatory requirements and guidelines. This ensures that products remain safe, effective, and compliant with relevant regulations.
In the context of collagen growth, researchers have investigated the potential of **Radiesse** to stimulate collagen production in various studies. While some data suggests that calcium hydroxyapatite fillers like **Radiesse** may promote collagen synthesis, the evidence is not yet conclusive.
More research is needed to fully understand the role of **Radiesse** in stimulating collagen growth and to clarify its potential benefits for this application.
The process of obtaining **regulatory approval** and gathering **safety data** is crucial for any medical device, including fillers like Radiesse. The goal of these efforts is to ensure that the product is safe and effective for its intended use.
For cosmetic fillers like Radiesse, which is a type of calcium hydroxylapatite, the **FDA clearance** process involves several steps. First, the manufacturer must submit an application to the FDA, including clinical trial data and other supporting documents.
The FDA then conducts a thorough review of the submission, evaluating factors such as the product’s ingredients, manufacturing process, and potential risks or side effects. This review may involve additional testing or inspections of the manufacturing facility.
Once the FDA has deemed the product satisfactory, it will issue an investigational device exemption (IDE) letter, which allows the manufacturer to begin marketing the product with certain restrictions.
For commercialization, Radiesse manufacturers must also gather additional data on the product’s performance in a larger patient population. This may involve conducting further clinical trials or collecting safety surveillance data from post-marketing reports.
The quality and depth of this **safety surveillance data** can significantly impact the FDA’s confidence in the product, which in turn can influence its approval status for future indications or applications.
Additionally, Radiesse manufacturers must also comply with other regulatory requirements, such as those related to Good Manufacturing Practice (GMP) and labeling. GMP standards ensure that the product is manufactured consistently and safely, while clear labeling can help educate healthcare professionals and patients about proper use and potential risks.
The relationship between Radiesse and collagen growth is also an area of interest for regulatory bodies. While some studies have suggested that calcium hydroxylapatite fillers like Radiesse may stimulate collagen production, these findings are not universally accepted.
As a result, the FDA has required additional clinical trials to fully assess the product’s efficacy and safety in promoting collagen growth. These trials have yielded mixed results, leading to ongoing debate among healthcare professionals and regulatory experts.
Despite the controversy surrounding Radiesse and collagen growth, the product remains popular among aesthetic practitioners for its versatility and established track record of safety and effectiveness.
However, as new evidence emerges on this topic, it is likely that regulatory bodies will reassess their stance on Radiesse’s indications and applications. In the meantime, manufacturers must prioritize ongoing monitoring and evaluation to ensure continued compliance with regulatory requirements and patient safety standards.
The interplay between **regulatory approval**, safety data, and FDA clearance is critical for any medical product seeking to demonstrate its value to both patients and payers. By understanding the complexities of this process, manufacturers can better navigate the ever-changing landscape of medical regulation and advance their products’ potential benefits.
Radiesse, a dermal filler composed of calcium hydroxylapatite microspheres and a gelling agent, has been approved by the US FDA for the treatment of nasolabial folds, marionette lines, and other facial wrinkles.
One of the key concerns about Radiesse is whether it stimulates collagen growth or merely fills in existing wrinkles. While some dermal fillers, such as collagen-based products, are known to stimulate collagen production over time, Radiesse works differently.
- Radiesse’s mechanism of action involves the introduction of calcium hydroxylapatite microspheres into the dermis, where they dissolve and release calcium ions. These ions stimulate fibroblasts, which produce new collagen and other matrix components.
- This process is thought to lead to a gradual increase in collagen production over several months, potentially contributing to longer-lasting results.
However, the FDA’s approval of Radiesse for long-term use has not necessarily been interpreted as evidence that it grows new collagen. Rather, their endorsement suggests that the product is safe and effective when used appropriately.
- The FDA’s review process involves a thorough evaluation of the product’s safety and efficacy in clinical trials.
- As part of this evaluation, the FDA examines the results of studies to assess the product’s ability to induce collagen growth, as well as its impact on other factors such as skin texture, facial contours, and patient satisfaction.
From a regulatory standpoint, the FDA has deemed Radiesse safe for use in treating wrinkles when administered according to recommended guidelines. This means that the agency is confident that the benefits of the product outweigh any potential risks or side effects.
It’s worth noting, however, that the exact mechanisms by which Radiesse works and whether it truly stimulates collagen growth are still not fully understood. Further research may be necessary to clarify these points and provide a more complete understanding of how this dermal filler operates.
In any case, for those interested in pursuing Radiesse treatment, it’s essential to discuss the product with a qualified healthcare professional who can review your individual needs, medical history, and expectations.
Radiesse, a dermal filler made from radioactive calcium hydroxylapatite, requires regulatory approval before being marketed and sold for cosmetic use.
The regulatory process involves submitting preclinical data to the U.S. Food and Drug Administration (FDA) as part of a 510(k) clearance application, which compares Radiesse to existing approved products.
Additionally, manufacturers must conduct clinical trials to demonstrate the safety and efficacy of Radiesse for specific indications, such as facial lipoatrophy or lip augmentation.
The results of these trials are used to create a Safety Data Sheet (SDS) that outlines the potential hazards associated with using Radiesse.
Regulatory agencies require manufacturers to report adverse reactions and other safety data in real-time, which is then added to the SDS.
The FDA also conducts inspections of manufacturing facilities to ensure compliance with Good Manufacturing Practices (GMPs) and that products meet quality standards.
Furthermore, Radiesse must be labeled with warning statements regarding potential side effects such as pain, swelling, bruising, or redness at the injection site.
The Safety Data Sheet includes information on proper handling, storage, and disposal of Radiesse, as well as recommended post-injection care to minimize risks.
In terms of safety profile, regulatory agencies review data on Radiesse’s toxicity, allergenic potential, and long-term effects, such as fibrosis or necrosis, in animal studies or clinical trials.
The FDA also evaluates the efficacy of Radiesse in treating specific conditions, ensuring that it meets established standards for approval.
Manufacturer-sponsored studies are required to evaluate the safety and tolerability of Radiesse over an extended period, typically several years after initial approval.
Regulatory agencies consider data from these long-term studies when deciding whether to extend indications or authorize new uses for Radiesse.
The FDA also monitors post-approval surveillance to detect any potential risks or side effects that may arise over time.
Radioss, the manufacturer of Radiesse, is responsible for updating the Safety Data Sheet and other regulatory documents as necessary to reflect any changes in the product’s safety profile or indications.
Radiesse, a dermal filler composed of *hyaluronic acid* and *calcium hydroxylapatite*, has been subject to rigorous testing and regulatory approval processes to ensure its safety and efficacy.
The development of Radiesse involved extensive clinical trials to assess its safety and effectiveness in promoting collagen growth. The results of these studies were submitted to regulatory authorities, including the US Food and Drug Administration (FDA), for review and approval.
In 2006, the FDA approved Radiesse for the treatment of moderate to severe facial folds and wrinkles, specifically targeting nasolabial folds. The agency evaluated the data from clinical trials, which demonstrated that Radiesse could stimulate collagen growth, leading to sustained results over time.
The safety profile of Radiesse was also thoroughly evaluated, with monitoring of potential side effects such as *inflammatory reactions*, *allergic responses*, and *vascular occlusions*. The FDA deemed the benefits of Radiesse to outweigh the risks, paving the way for its commercialization.
As part of its regulatory approval, the manufacturer submitted a Packaging Insert**, which provides comprehensive information on the product’s ingredients, usage instructions, and potential side effects. This document serves as a crucial resource for healthcare professionals and patients alike, ensuring that Radiesse is used safely and effectively.
Regarding common side effects, users of Radiesse may experience *mild to moderate swelling**, *redness**, or *itching** at the injection site. In rare cases, more severe reactions such as angioedema, a potentially life-threatening condition, may occur. It’s essential for patients to report any unusual symptoms or concerns to their healthcare provider.
To address these potential side effects, manufacturers often provide *Patient Information Sheets**, which outline the possible risks and benefits of Radiesse treatment. These documents are designed to inform patients about the product’s characteristics, usage guidelines, and necessary precautions.
The FDA also requires manufacturers to conduct ongoing post-marketing surveillance**, which involves monitoring the safety of approved products after they’ve entered the market. This allows for early detection and mitigation of any new or emerging side effects.
Additionally, many healthcare providers participate in continuous education and training programs to stay up-to-date on the latest research and guidelines regarding dermal fillers like Radiesse. These efforts help ensure that patients receive informed care and optimal results from their treatments.
Ultimately, the combination of rigorous testing, regulatory approval, and ongoing monitoring enables healthcare professionals and patients to make informed decisions about using Radiesse for facial rejuvenation treatments, including its potential to stimulate collagen growth.
Radiesse, a popular dermal filler used to treat various aesthetic concerns such as nasolabial folds, marionette lines, and lip augmentation, has been the subject of much scrutiny in recent years.
One of the primary questions surrounding Radiesse is whether it truly stimulates collagen growth, or if its effects are solely temporary.
A study published by the European Society of Cosmetic Surgery and Aesthetic Medicine sheds some light on this topic. However, before diving into the specifics of this study, it’s essential to understand the regulatory approval process for cosmetic products like Radiesse.
Radiess is approved by the US FDA as a dermal filler for the treatment of nasolabial folds, marionette lines, and lip augmentation. To receive this approval, the manufacturer must demonstrate that the product is safe and effective in reducing the appearance of these facial concerns.
The safety and efficacy of Radiesse were established through a comprehensive clinical trial program, which included multiple studies examining its effects on various patient populations.
As part of its regulatory approval process, the manufacturer submitted safety data from these trials to support its claims. This safety data typically includes information on common side effects, such as swelling, redness, and bruising, as well as rare but serious complications.
The study by the European Society of Cosmetic Surgery and Aesthetic Medicine found that Radiesse is associated with some common side effects, including swelling, redness, and bruising. However, it’s essential to note that these side effects are generally mild and temporary.
Furthermore, serious complications with Radiesse, such as infection or allergic reactions, are rare.
While Radiesse may not directly stimulate collagen growth in the same way that some other dermal fillers do, it can still provide long-term results by encouraging the body to produce its own natural collagen.
This phenomenon is often referred to as “stimulating” or “eliciting” collagen production, rather than actively producing it. In the case of Radiesse, its unique formula is designed to stimulate an inflammatory response in the body, which can lead to increased collagen synthesis over time.
However, the extent to which Radiesse stimulates collagen growth versus providing temporary results has been a topic of debate among dermatologists and plastic surgeons. Some studies suggest that while Radiesse may not be as effective at stimulating collagen production as some other dermal fillers, it can still provide significant long-term benefits.
In any case, the safety data provided by manufacturers such as Merz Aesthetics (the maker of Radiesse) plays a crucial role in reassuring regulatory agencies and healthcare professionals about the potential risks associated with using this product.
This information is essential for establishing the efficacy and safety profile of Radiesse, and can inform treatment decisions for patients considering this dermal filler.
Read more about Democracy Defense Coalition here. Read more about The Fine Nanny here. Read more about Elizabeth Joy Photo here. Read more about Raindrops and Ribbons here.
- What Not To Do After Tear Trough Filler - May 31, 2025
- Intersectionality In LGBTQ+ Dating And Relationships - May 30, 2025
- Light Eyes Ultra – Dark Circles Treatment Near Kempton Park, Surrey - May 30, 2025
